Chain Transfer Reactions
in Cationic Polymerization
Chain transfer reactions are important termination reactions in cationic polymerization which affect the final molecular weight of the polymer and may also result in branching. These reactions occur readily because the β-hydrogen of the carbenium ion is strongly positively polarized due to hyperconjugation.1 Or in other words, not all of the positive charge resides on the positively charged carbon atom. Consequently, carbenium ions easily participate in many side reactions such as chain transfer, termination and/or isomerization.
There are several types of chain transfer reactions; three very common reactions are:
Proton transfer to monomer: a β-proton is transfered to a monomer, which becomes the new active growth center, while the terminal carbon atom simultaneously forms an inactive double bond with one of its neighboring atoms.
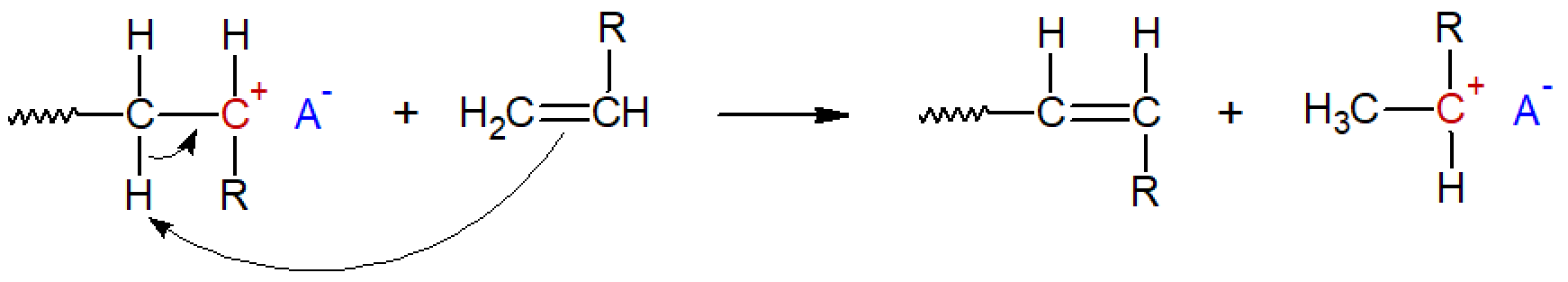
Hydride ion transfer from monomer: another type of chain transfer involves the transfer of a hydride ion from a monomer to the chain growth center which results in a saturated end group. The vinyl cation simultaneously formed by the hydride transfer is less stable than the secondary/tertiary carbocation and thus is more reactive.
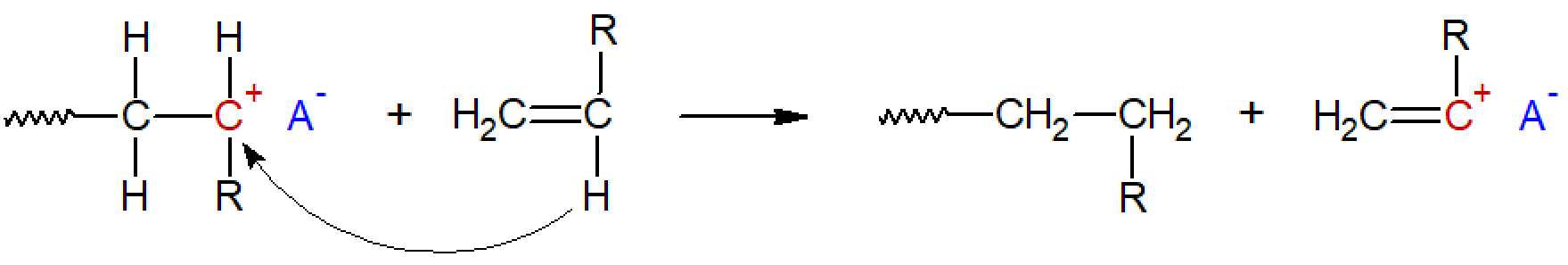
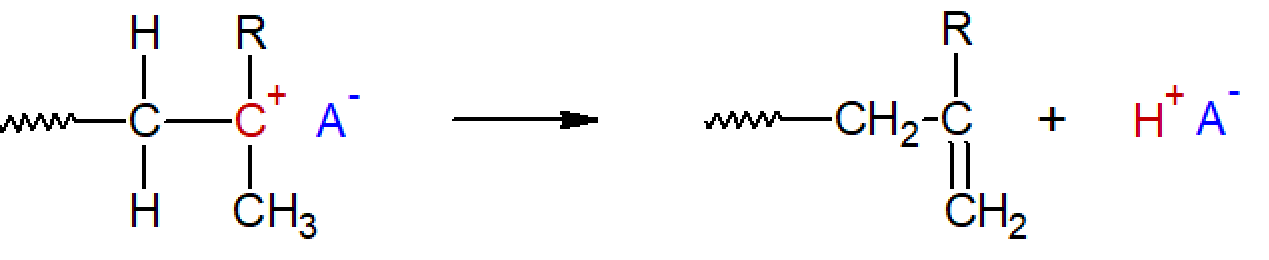
Proton transfer to counterion: This process involves the transfer of a proton from the carbocation to the counterion. This reaction is also known as spontaneous termination. Unlike the other proton transfer processes, this reaction terminates the chain growth. However, chain growth of the inactive polymer can be readily reinitiated because the polymer possesses a terminal double bond.
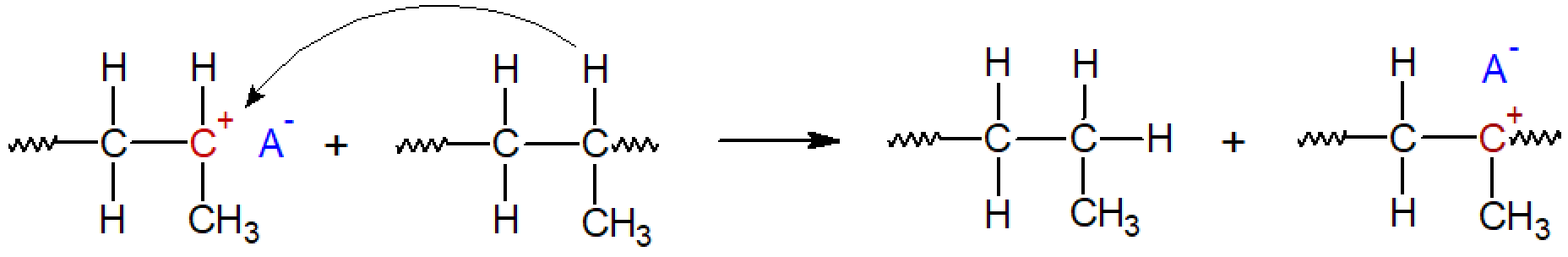
Intermolecular hydride ion transfer to polymer: It is also possible that a hydride ion of another polymer chain is transferred to the active growth center of the propagating polymer chain. This reaction not only lowers the molecular weight but also leads to chain branching:
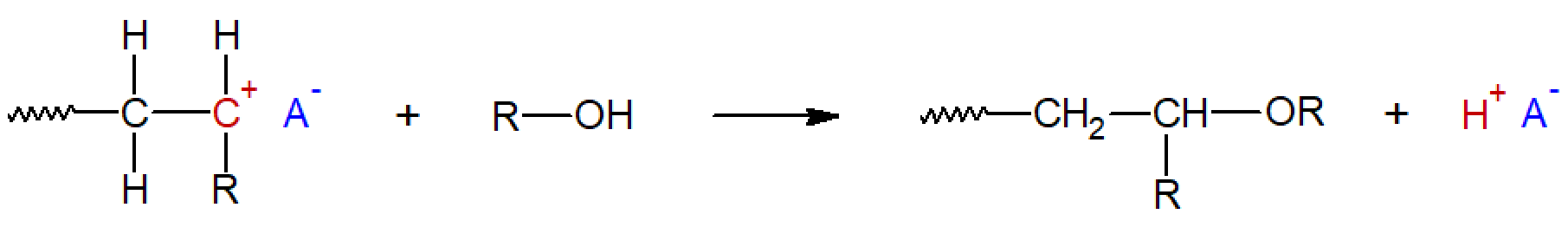
Many other chain transfer reactions are possible. For example, chain transfer to a solvent or to an impurity present in the mixture can occur, or a transfer agent might be intentionally added to the system. Typical examples of such transfer agents are water, alcohols and acids. These compounds can transfer a HO, RO, or RCOO anion to the chain carbocation which terminates the chain growth. For example:
Notes
-
Hyperconjugation is the stabilising interaction of electrons of a sigma orbital (C-C, C-H) with an adjacent empty or partially filled non-bonding orbital which creates an extended molecular orbital of increased stability. This concept can also be applied to carbenium ions and radicals.